

Forest Ho-Chen '22
Rate Laws
In chemistry, kinetics studies the rate of reactions. The rate of a reaction is often dependent on the concentrations of the reactants. This dependence is shown as a rate law, which includes the concentrations of the reactants raised to powers and multiplied together. Rate laws also include a rate constant, k, which depends on the temperature and the substances of the reaction.
When there is only one reactant, we can have integrated rate laws, which are found by writing a first-order differential equation, separating the variables, and then integrating both sides of the equation, then setting the limits for the given conditions.

In the Time Travel Comic, No Name Zinwolf and Annie Bertha use two equations with four rate constants in order to show the relationship between ΔH and K. Where did those equations come from?
The Arrhenius Equation gives us a formula for how the rate constant depends on the activation energy of the reaction and the temperature of the reaction. Using two of this equation for the same reaction at different temperatures, we can get that equation found in the comic:
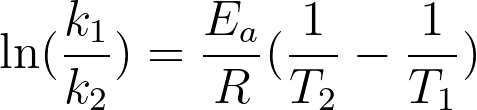
This equation looks similar to other equations in science:

The Arrhenius Equation reflects the collision theory. With a higher temperature, the molecules can move around more, allowing for more collisions. If the reaction has a lower activation energy, a higher fraction of the collisions will be above the activation energy. This is why the rate constant increases with a higher temperature and decreases with a higher activation energy.